Abstract
Palbociclib is a reversible cyclin dependent kinase 4 and 6 (CDK4/6) inhibitor that is approved for the treatment of hormone-receptor positive metastatic breast cancer (ER+/HER2- MBC). CDK4/6 inhibitors exert their anti-tumor effect by preventing the G1 to S phase cell cycle transition, resulting in G1 cell cycle arrest. The most commonly reported hematologic side effect of palbociclib is neutropenia. Here we report 3 cases of reversible macrocytic anemia with dysplastic changes as a result of palbociclib use. Next, we present a retrospective study of 32 patients treated with palbociclib. Finally, CDK4 and CDK6 levels were measured in CD34+ stem/progenitor cells (HSPC) from myelodysplastic syndrome (MDS) patients and compared to healthy controls.
Methods & Results
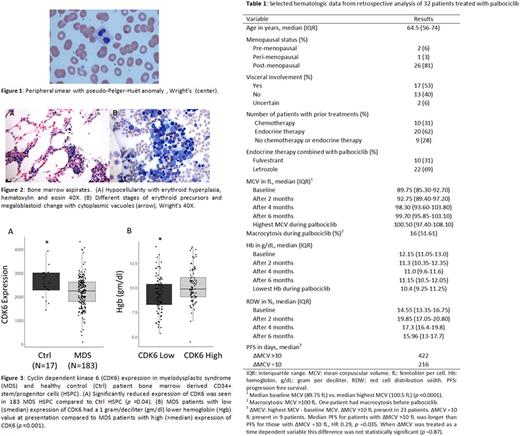
Four months into starting treatment with palbociclib plus an aromatase inhibitor for refractory ER+/HER2- MBC, 3 female patients developed macrocytic anemia, with a mean corpuscular volume (MCV) of >100 femtoliters per cell (fL) and a decrease in hemoglobin (Hb) by 2 grams per deciliter (g/dL). Work-up for macrocytosis was unrevealing except for peripheral blood smears showing dysplastic changes including pseudo-Pelger-Huёt anomaly, suggestive of MDS (see Figure (Fig) 1). Bone marrow aspirates were obtained, which also showed morphology similar to MDS including hypocellular marrow (10% cellularity), dysplastic erythroid forms including binucleated forms and nuclear budding, and small hypolobated megakaryocytes (see Fig 2). Cytogenetic, molecular, and flow analyses were normal. Patients were managed expectantly, and when palbociclib was discontinued for breast cancer progression, the macrocytic anemia resolved to baseline Hb and MCV values within 4 months of stopping therapy.
A retrospective study to assess hematological characteristics of 32 patients with ER+/HER2- MBC treated with palbociclib for more than 3 months between 1/1/2015 to 3/1/2017 was performed. Statistical analysis included Wilcoxon signed-rank test and Kaplan Meier estimator. Median age was 65 years and 10 (31%) patients had received prior chemotherapy (see Table 1). Median baseline MCV was 89.75 fL compared to 100.5 fL post palbociclib (p <0.0001), and 16 (52%) patients developed macrocytosis (MCV >100 fL). The median progression-free-survival (PFS) for patients who developed a significant MCV increase (highest MCV >10 fL from baseline MCV) was 422 days compared to 216 days for those without a significant MCV increase (highest MCV ≤10 fL from baseline MCV) (HR 0.29, p=0.035). However, this PFS difference was not seen when the change in MCV was analyzed as a time-dependent variable.
As palbociclib treatment appears to induce hematologic and bone marrow changes phenotypically similar to MDS, we examined whether alterations in CDK4/6 expression is observed in human MDS. We evaluated CDK4 and CDK6 expression by qRT-PCR in 183 samples from MDS patient-derived HSPC and 17 healthy marrow control HSPC and found that CDK6, but not CDK4, expression was significantly decreased in MDS HSPC when compared to healthy HSPC (p=0.04) (see Fig 3). We then evaluated MDS phenotype by high (>median) and low (≤median) CDK6 expression, and found that patients with low CDK6 expression had a 1 g/dL lower Hb at presentation when compared to those with high CDK6 expression (p=0.001). Further, when healthy marrow HSPC were treated with palbociclib in vitro, we observed a dose dependent decrease in erythroid colony formation and maturation, replicating the anemia phenotype seen in patients.
In conclusion, palbociclib treatment can cause a reversible macrocytic and dysplastic anemia. Macrocytosis is a potential indicator of in vivo CDK4/6 inhibition that could be associated with increased PFS in patients with ER+/HER2- MBC who are treated with palbociclib. Low CDK6 expression in MDS correlates with a worsened anemia phenotype. Further clinical studies with larger sample sizes are needed. Continued safety monitoring of patients on palbociclib is suggested.
Steidl: Aileron Therapeutics: Consultancy, Research Funding; GlaxoSmithKline: Research Funding; Novartis: Research Funding; Celgene: Consultancy; Bayer Healthcare: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal